Information
News
Technology
PUREfrex® -based on the PURE system-
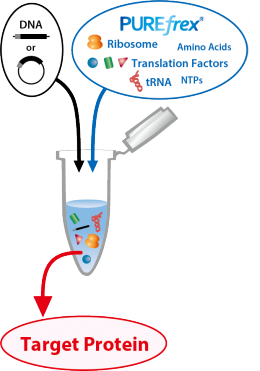
PUREfrex® kit is a reconstituted in vitro Coupled Transcription/Translation Systems, completely different from an E.coli extract S30 system.
By adding DNA or mRNA that encodes the target protein to the reaction solution, proteins can be synthesized easily and quickly without using living cells.
Products
Cell-free Protein Synthesis Kits

A product lineup of reconstituted cell-free protein synthesis kits developed based on the PURE system technology.
Supplements - Chaperones -

Optional kit for adding PUREfrex® to assist proper folding of protein.
Supplements - Forming disulfide bonds -

Optional kit for adding PUREfrex® to assist protein disulfide bond formation.
Other proteins

Optional kit for adding PUREfrex® to assist synthesis of proteins with rare sequences.
PUREfrex® Custom - Made to order –

This service allows you to freely customize the composition of each solution included in the PUREfrex® catalog products.









