We synthesized vtPA and AppA as a model protein, which has multiple disulfide bonds, for this experiment. About the model proteins, please see below for the detailed information. The model proteins were synthesized at different concentrations of E.coli DsbC attached to PUREfrex™ SS kit (# PF002). According to the protocol, please visit our web site, the template DNA of vtPA and AppA were added to the reaction mixture and incubated at 37 ℃ for 4 hours at concentrations of DsbC from 0 – 750 μg/mL of reaction mixture. For dilution of DsbC, Dilution Buffer attached to the kit (# PF002) was used.
After synthesizing of vtPA and AppA, their activity was assayed with their substrate.
These results are showed in Fig. 1. The activity was measured and compared as relative value. The maximum activity was set to 100.
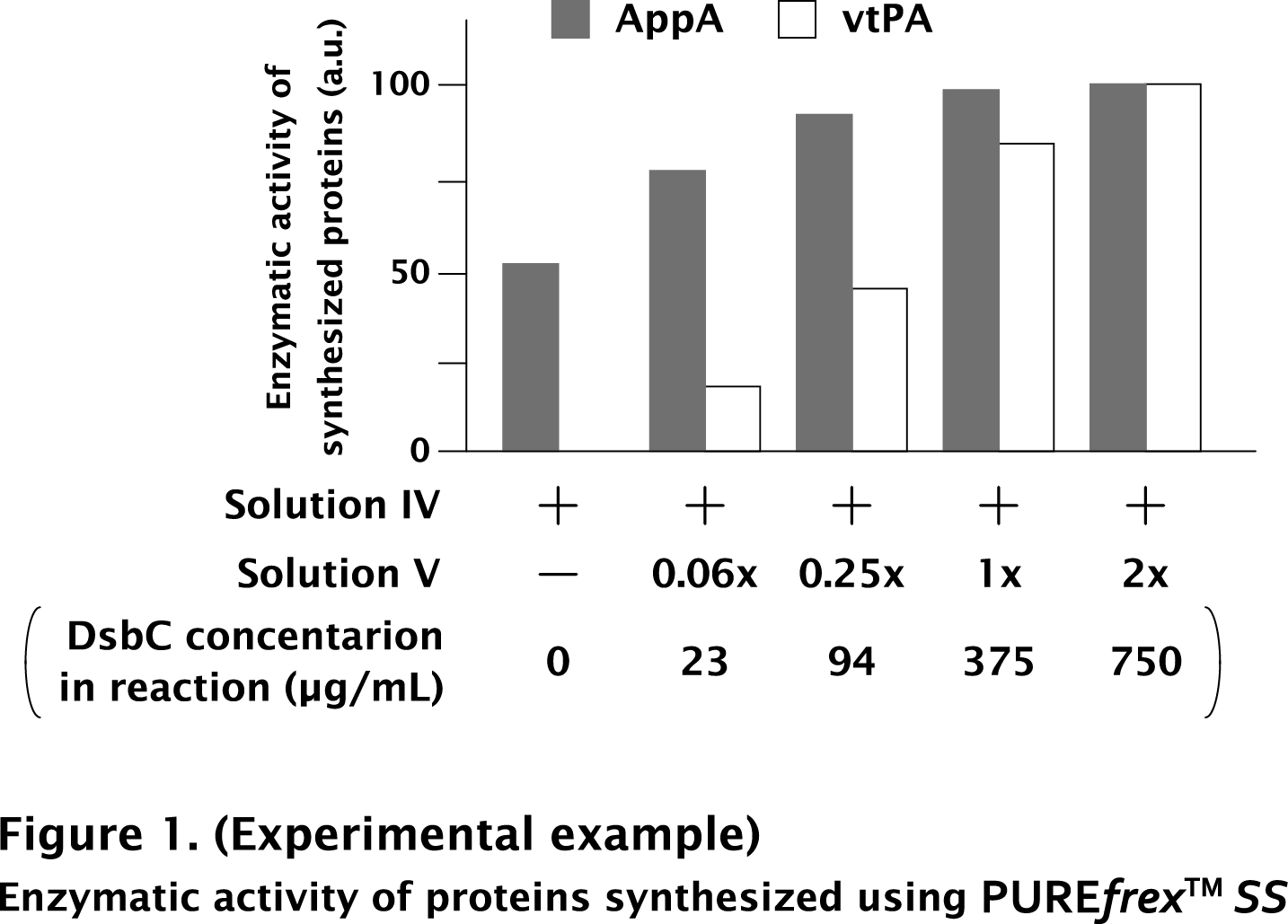
The amount of DsbC to 50 μL reaction mixture
Solution V attached to the kit (PF002) was used as DsbC
23 µg/mL : 2.5 µL of Solution V diluted 16-fold
94 µg/mL : 2.5 µL of Solution V diluted 4-fold
375 µg/mL : 2.5 µL of Solution V
750 µg/mL : 5 µL of Solution V
As a result, AppA showed 50 % of its activity even without DsbC. On the other hand, vtPA required DsbC for its activity. It indicates that the optimum concentration of DsbC is different in each protein.
Model proteins
vtPA(truncated version of tissue plasminogen activator)is the protease domain and has 9 of SS-bond showing 8 of them bound between non-consecutive cysteine.
AppA is an acid phosphatase from E.coli and has 5 of SS-bond showing 1 of them bound between non-consecutive cysteine.

