In PUREfrex®, a reducing reagent is added to mimic the cytosol environment and allow the transcription and translation reactions in a reduced state. However, when DTT was used as a reducing reagent and the reaction solution was incubated for a long time, it was observed that the reaction solution was in an oxidized state.
Therefore, we used the different type of reducing reagent to check its effect on the solution of PUREfrex® and its effect on the activity of some proteins.
1. Reducing reagent
The following is a summary of the types and structures of the reducing reagents used in this experiment. Abbreviations are given in parentheses.
2. Influence on reaction mixture
After prolonged incubation of the PUREfrex® reaction mixture, the reaction mixture became oxidized, and for some factors, the shift in the bands was observed when analyzed by SDS-PAGE under non-reducing conditions.
2-1. Redox state in the reaction mixture
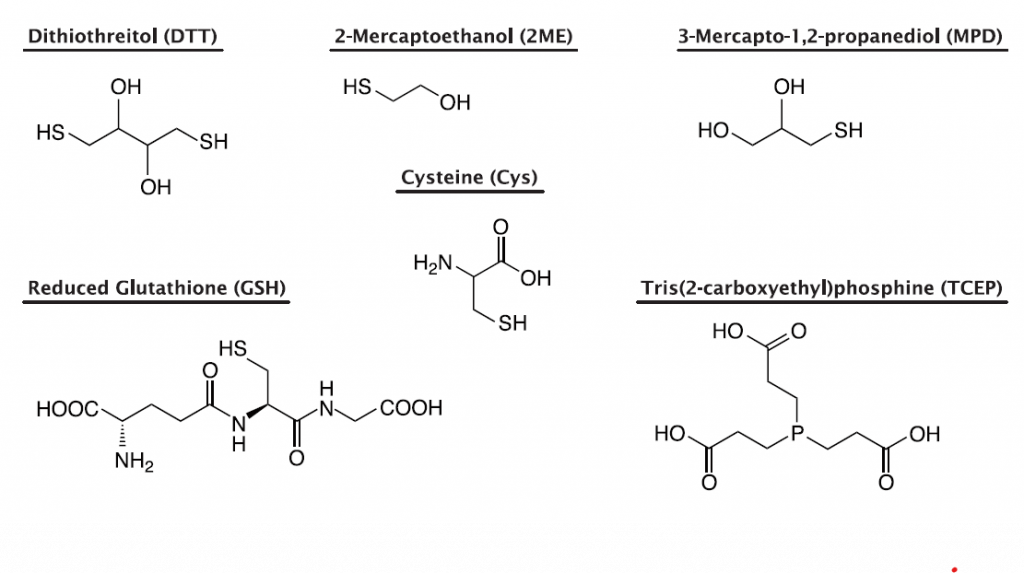
Solution I of PUREfrex® containing buffers, amino acids, NTPs, tRNAs, and substrates of enzymes was prepared without reducing reagents, and different reducing reagents were added and incubated for 24 hours, after which the SH groups were analyzed by Ellman’s assay.
As a result, it was observed that the SH group decreased after incubation when the reducing reagents in Solution I were DTT and TCEP.
When GSH, Cys, and 2ME were added as reducing agents in Solution I and incubated, there was almost no decrease in SH groups, and the reaction solution did not become oxidized state.
2-2. Oxidation of several factors
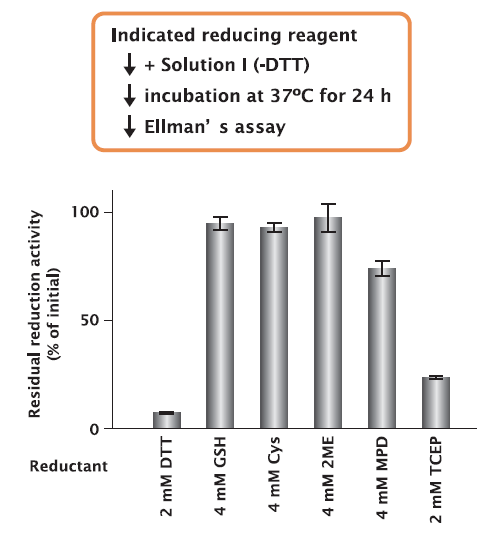
Solution II of PUREfrex® , which contains proteinous factors for transcription, translation, etc., was incubated with Solution I, which was prepared to contain different reducing reagents, and then analyzed by non-reducing SDS-PAGE.
The results showed that when Solution I containing DTT and Cys was used, some factors were oxidized and the migration distances was shifted.
On the other hand, when Solution I containing GSH, 2ME, and MPD was used, no band shift of factors was observed.
3. Synthesis of proteins without a disulfide bond
Proteins, disulfide bonds are not essential, were synthesized in reaction solutions including different reducing reagents, and their activities or non-reducing SDS-PAGE protein patterns of synthesized products were analyzed.
3-1. GFP
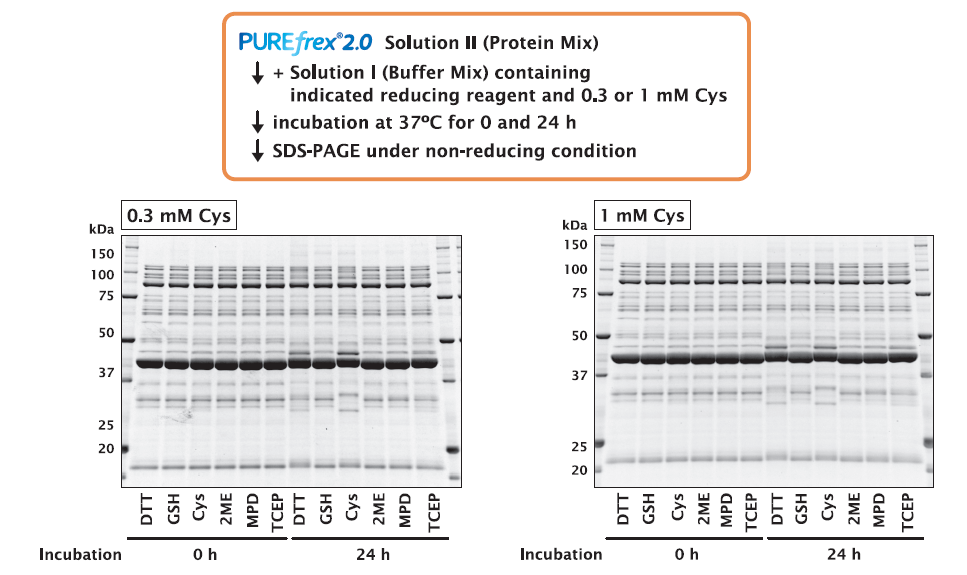
GFP was synthesized in the presence of different reducing reagents for 4 and 24 hours, and the activity of the synthesized GFP was compared by fluorescence intensity.
As a result, there was no difference in the fluorescence intensity depending on the type of reducing reagent. This indicates that the activity of GFP is not affected by the type of reducing reagent.
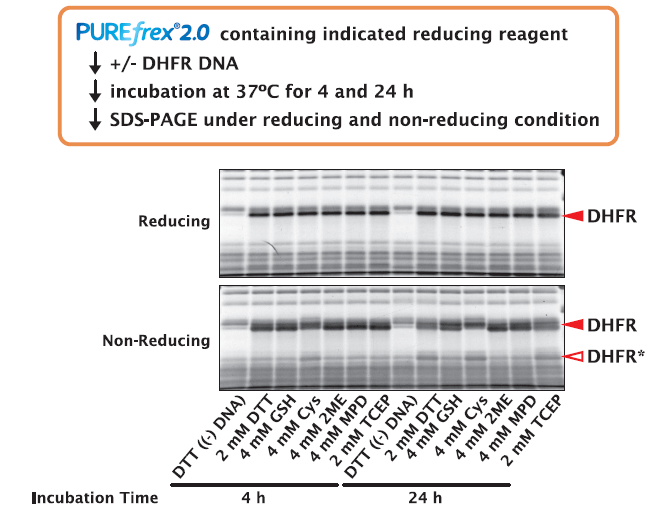
3-2. DHFR
DHFR was synthesized in the presence of different reducing reagents for 4 and 24 hours. The synthesized DHFR were analyzed by SDS-PAGE under reducing and non-Reducing condition to see if there was any shift in the migration distances of DHFR.
The results showed that there was no difference in the amount of DHFR synthesized by different reducing reagents on SDS-PAGE under reducing condition.
However, when analyzed by non-reducing SDS-PAGE, DHFR was oxidized and a band shift was observed when DTT, Cys, and TCEP were included in the reaction mixture (labeled DHFR* in the figure).
When GSH, 2ME, and MPD were included in the reaction mixture, little oxidized DHFR was observed.
4. Synthesis of proteins containing disulfide bonds
Proteins, disulfide bonds are essential for the activity, were synthesized in reaction mixture including different reducing reagents, and their activities and non-reducing SDS-PAGE protein patterns of synthesized products were analyzed.
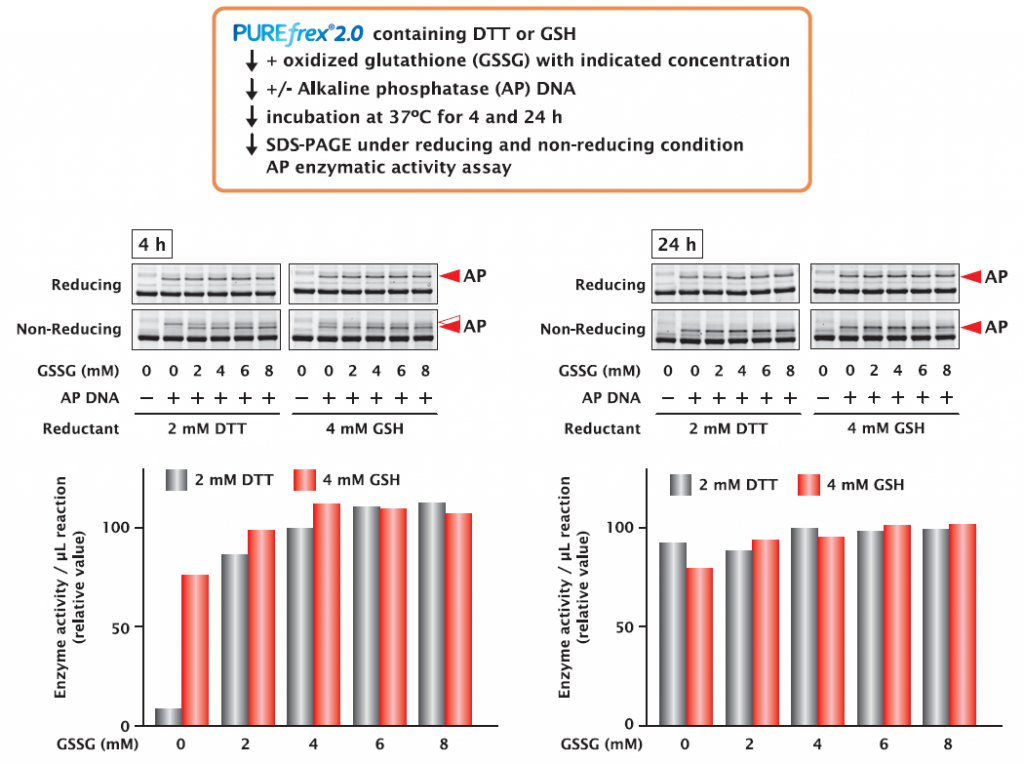
4-1. Alkaline phosphatase (AP)
AP was synthesized under different concentrations of DTT and GSH as reducing reagents and GSSG as oxidizing reagent for 4 and 24 hours. The synthesized APs were compared for their activities and band shifts were confirmed by SDS-PAGE.
As a result, in the case of a 4-hour incubation, analysis of non-reducing SDS-PAGE showed that when DTT was used as the reducing agent, the AP existed almost in the reduced form and had low activity under the conditions without GSSG.
On the other hand, when GSH was used as a reducing agent, the AP existed in the oxidized form and showed activity even in the absence of GSSG.
These results indicate that AP is a protein that can form disulfide bonds in reaction mixture without oxidant (GSSG), either by selecting GSH as a reducing reagent or by prolonged incubation.
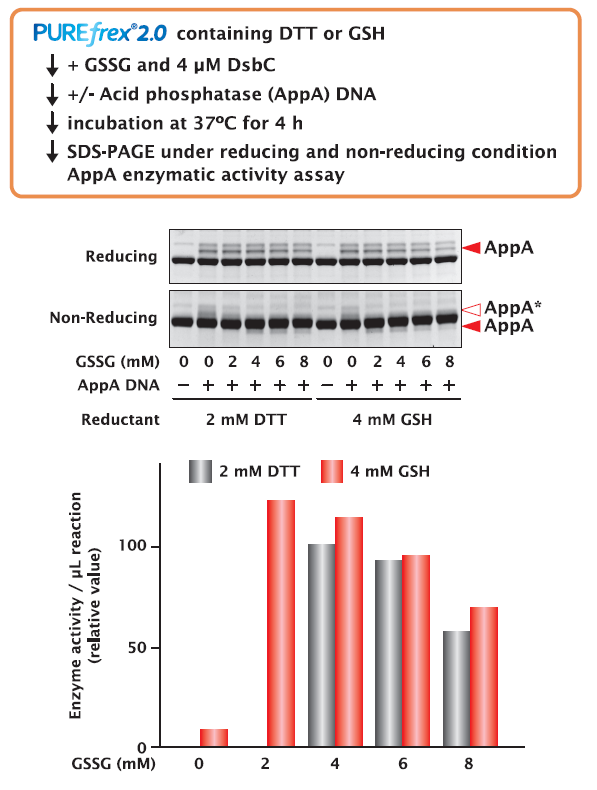
4-2. Acid phosphatase (AppA)
AppA was synthesized under different concentrations of DTT and GSH as reducing reagents and GSSG as oxidizing reagent for 4 hours. The synthesized AppAs were compared for their activities and band shifts were confirmed by SDS-PAGE.
As a result, analysis of the non-reducing SDS-PAGE showed that the reduced AppA (labeled AppA* in the figure) was present in all conditions, but the higher the ratio of oxidized AppA (red triangle) present, the higher the activity.
In the case of AppA, we found that GSH tended to form disulfide bonds more readily than DTT.
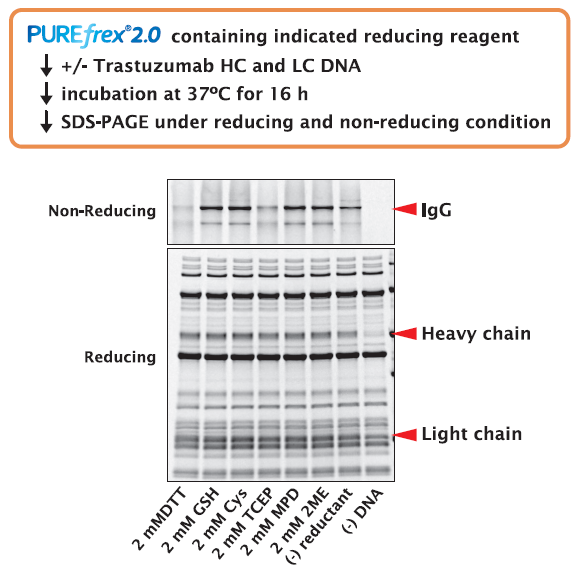
4-3. IgG (Trastuzumab)
In the presence of different reducing reagents, template DNAs of the H- and L-chain of trastuzumab were synthesized simultaneously in the reaction mixture, and after incubation for 16 hours, the formation of the IgG structure was confirmed by analysis of non-reducing SDS-PAGE.
As a result, there was no difference in the amount of both H and L chains synthesized depending on the type of reducing reagent (comparison by reducing SDS-PAGE analysis).
However, the efficiency of IgG formation differed depending on the type of reducing reagent, and when DTT and TCEP were used, IgG was hardly formed.matom
5. Conclusion
It was observed that depending on the type of reducing reagent, the PUREfrex® reaction solution itself, as well as some foctors, tended to oxidize.
For the proteins, disulfide bonds are not essential, there was no significant effect of the type of reducing reagent after 4 hours incubation, but after 24 hours incubation, some proteins showed oxidized form depending on the type of reducing reagent.
For proteins, disulfide bonds are essential for the activity, it was confirmed that the optimal conditions for disulfide bond formation differed depending on the protein. Therefore, it is necessary to optimize the mixing ratio of reducing and oxidizing reagents for each protein.
In addition, when the formation of intermolecular disulfide bonds, such as in the case of IgG, a long incubation period is required for its formation, and furthermore, the efficiency of IgG formation varies depending on the type of reducing reagent.
Based on these results, we recommend optimizing the mixing conditions of the reducing and oxidizing reagents for the synthesis of proteins that require the formation of disulfide bonds. In particular, for proteins that require intermolecular disulfide bonds, it is recommended to consider the type of reducing agent and let it incubate for a longer time.
< Poster >
https://www.genefrontier.com/files/p17_PSSJ2017.pdf

